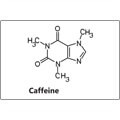
Organic compounds are basically compounds that contain carbon and hydrogen. The simplest organic compound is methane, which contains one carbon atom covalently bonded to four hydrogen atoms.
These compounds are called organic because they were once believed to all have been derived from living things, but that is not necessarily the case. There are millions of organic compounds that occur naturally or can be produced synthetically. Organic compounds are the basis for the molecules of life, like carbohydrates, fats (lipids), proteins, and nucleic acids. The main components of fossil fuels (petroleum and natural gas) are a class of organic compounds known as hydrocarbons.
Organic compounds are divided into two main groups: hydrocarbons, which comprise compounds that contain only carbon and hydrogen atoms, and a second type defined by a functional group that has replaced one of the hydrogen atoms in the molecule. Functional groups can be a single atom (like F, Cl, Br, or I) or a combination of atoms (NH2, OH, C=O, or SH).
Hydrocarbons can be further classified as:
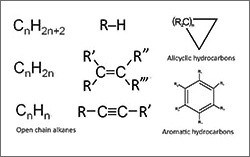
- Alkanes: saturated hydrocarbons that contain a linear or branched chain of only C-C and C-H bonds
- Alkenes: unsaturated hydrocarbons that contain doubly-bonded carbon atoms (C=C)
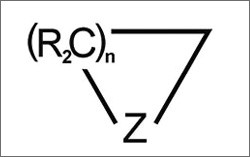
- Alkynes: unsaturated hydrocarbons with triply-bonded carbon atoms (–C≡C–) or their cyclic counterparts (carbon atoms that bond to form a ring structure)
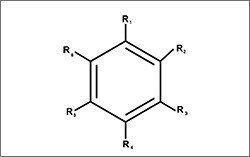
- Arenes: aromatic hydrocarbons that contain at least one benzene ring (a ring of six doubly bonded carbon atoms that exhibits unique chemical properties)
The chemical reactivity of organic molecules is influenced by the functional groups:
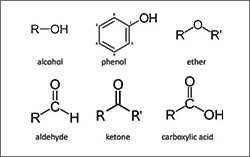
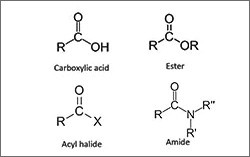
- Oxygen-containing groups (alcohols, ethers, aldehydes, ketones, carboxylic acids, and carboxylic acid esters)
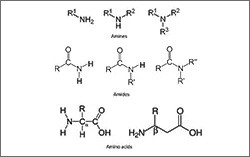
- Nitrogen-containing groups (amines, anilines, and amides)
- Sulfur-containing groups (thiols, thioethers, thioesters, and disulfides)
Carbon atoms can also bond with heteroatoms or other elements to form aliphatic or aromatic heterocyclic rings of varying sizes and reactivities.
Because of the almost infinite number of ways in which carbon can bond with carbon and other elements, it’s no surprise that about 95 percent of the millions of known compounds are organics with highly diverse structures. New organic compounds continue to be discovered and synthesized every day, adding to the already wide range of applications in all aspects of modern life — including medicine, pharmaceuticals, food, textiles, materials, and electronics.
Compounds or allotropes of carbon that contain only carbon atoms are classified as inorganic compounds and exhibit novel properties. This class of chemicals has a wide range of potentially useful applications and includes graphite, diamond, and the more recently discovered graphene, fullerenes, and other carbon nanotubes.